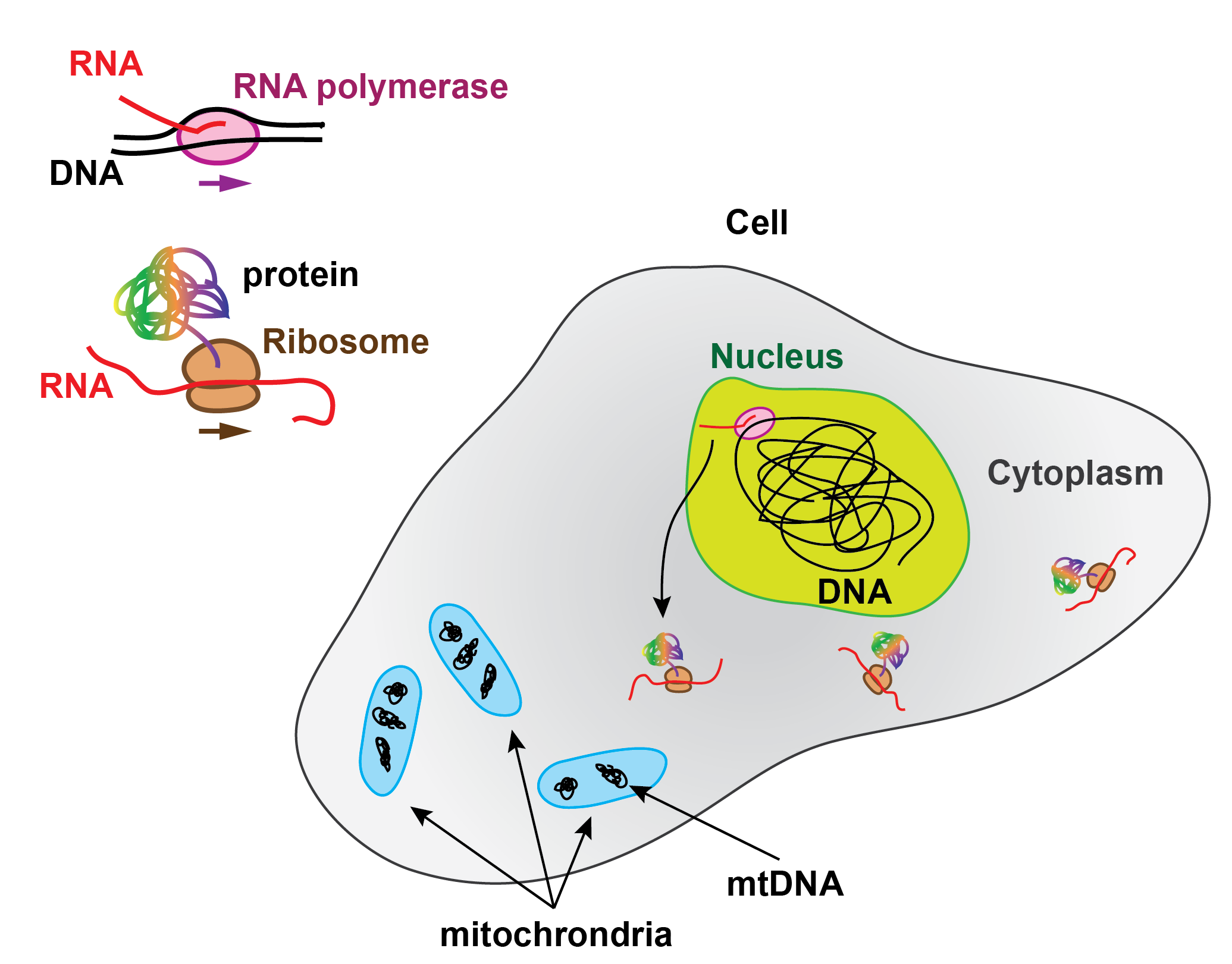
In the Central dogma of molecular biology, RNA production is the intermediate step for protein synthesis. The genes encoded in the DNA are read and transcribed by the RNA polymerases (RNAP) into RNA, part of which, called messenger RNA, is subsequently translated into proteins by the ribosomes.
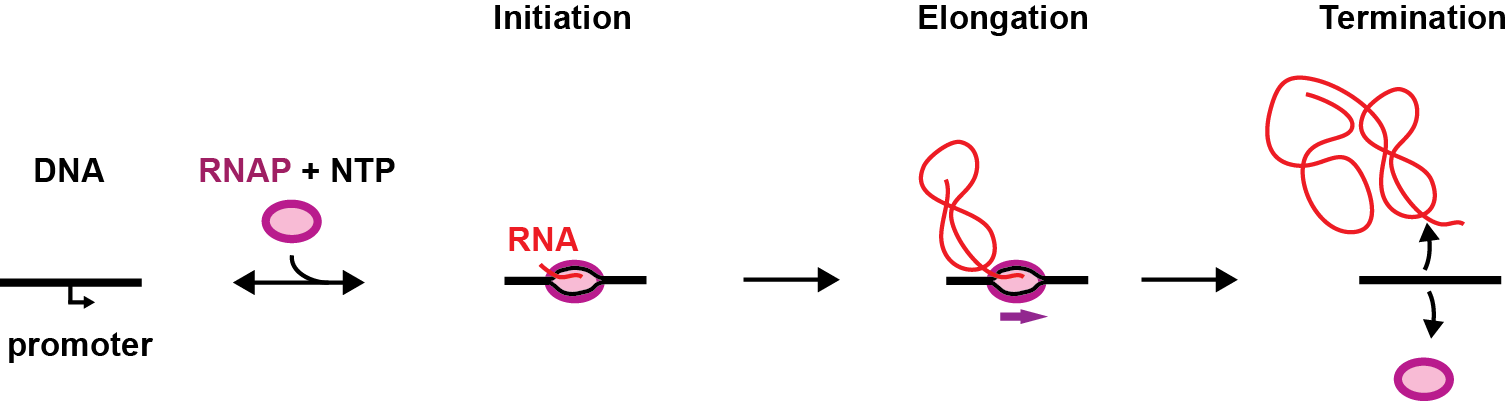
Being a key process in gene expression, transcription is therefore highly regulated in cells and is divided in three steps, i.e. initiation, elongation and termination.
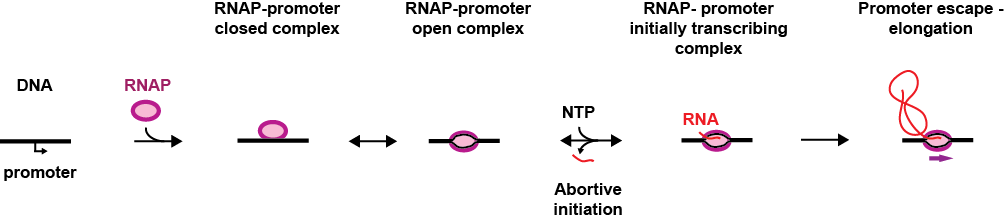
During initiation, the pre-initiation complex (PIC), formed by the RNAP and its associated factors, recognises the promoter, i.e. a DNA sequence that precedes the gene, and binds to it specifically, which forms the RNAP-promoter closed complex (RPc). The PIC subsequently opens the DNA promoter to make a transcription bubble, which forms the RNAP-promoter open complex (RPo) and places the RNAP active site at the transcription start site of the gene. In the presence of nucleotides (NTP), the RNAP initiates RNA synthesis (RPitc, itc: initially transcribing complex), which leads to either promoter escape towards elongation and dissociation from the PIC co-factors, or to abortive initiation, i.e. release of the nascent RNA, bringing the PIC back to the RPo stage. Interestingly, some promoters are rate-limiting for gene expression at the RPo stage, which is driven by promoter-PIC interactions, others at RPitc, driven by the initially transcribed sequence. Understanding the nature of these interactions and how they modulate promoter-based gene expression regulation is therefore of great importance.
As every single steps during transcription initiation is reversible and stochastic, transcription initiation study using classical biochemistry ensemble experiments provides only the averaged behaviour of a heterogeneous sample. Therefore, ensemble techniques cannot access the kinetics of every intermediates, and provide an accurate description of transcription initiation. Single molecule techniques, such as single molecule FRET and magnetic tweezers, have been very useful to decipher the complex branched biochemical pathways of transcription initiation using their ability to observe the dynamics of individual RNA polymerases, and therefore discovered, e.g. pauses of various biochemical origins that regulates initial transcription in bacteria.
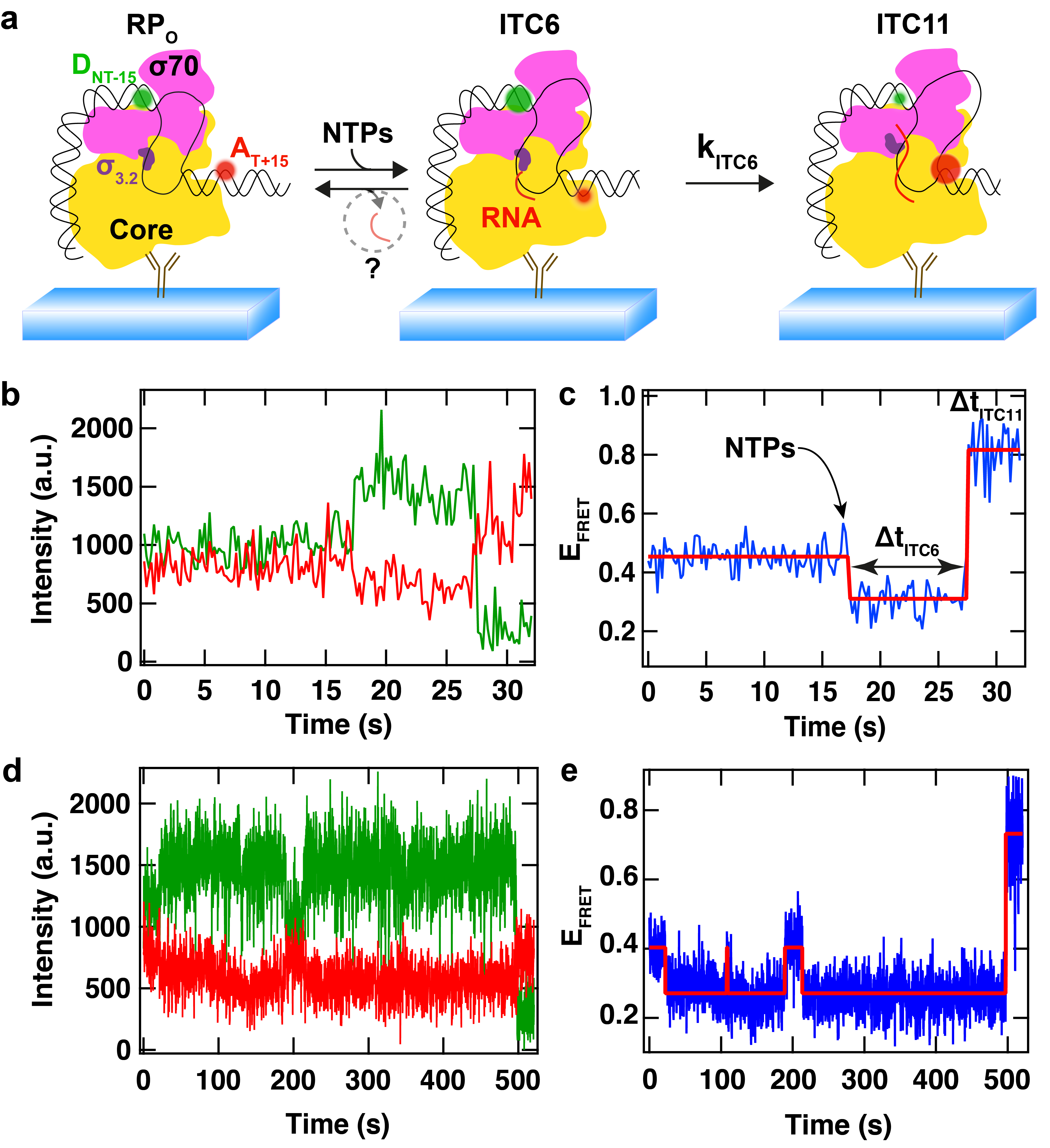
(a) Schematic of bacterial RNAP initial transcription study using single molecule FRET. The donor dye (D, green) and the acceptor dye (A, red) flank the transcription bubble. Upon NTP addition, the downstream DNA is scrunched in the RNAP, while the upstream DNA is static, which modifies the distance between the two dyes, leading to a change in FRET efficiency. (b) Fluorescence intensity of the donor (green) and of the acceptor (red) dyes. (c) FRET efficiency calculated from (b). The FRET efficiency (blue) first decreases upon addition of NTPs and the synthesis of the first 6 nucleotides of RNA (ITC6) by the RPitc, which pauses before extending the RNA to 11mer. The red step line is the Viterbi trace recovered from a Hidden Markov Model (HMM) to detect the changes in FRET efficiency. (d, e) Another FRET trace acquired in the same reaction conditions, though showing a completely different pausing behaviour. Adapted from Dulin et al., Nature Communications 2018.
In the Dulin lab, we investigate transcription initiation on various cellular systems, such as the human mitochondria transcription complex, using bespoke high throughput magnetic tweezers. We are currently developing a bespoke TIRF microscope for single molecule FRET experiments to complement our magnetic tweezers approach.
During elongation, it has been shown that the RNAP encounters various type of pauses that halt transcription. These pauses are important regulatory checkpoints for, e.g. transcription factor to bind the RNAP, to synchronise with co-transcriptional processes such as translation in bacteria. The high throughput capability of our magnetic tweezers assay offers the possibility to deeply probe transcription elongation and characterise rare – but relevant – biochemical states.
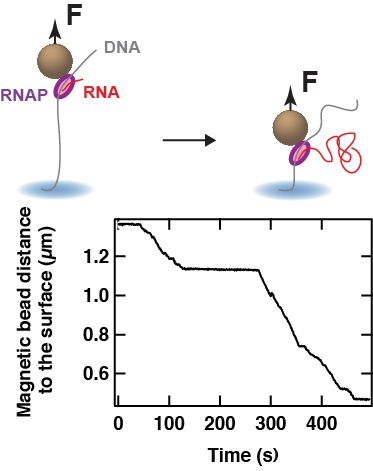
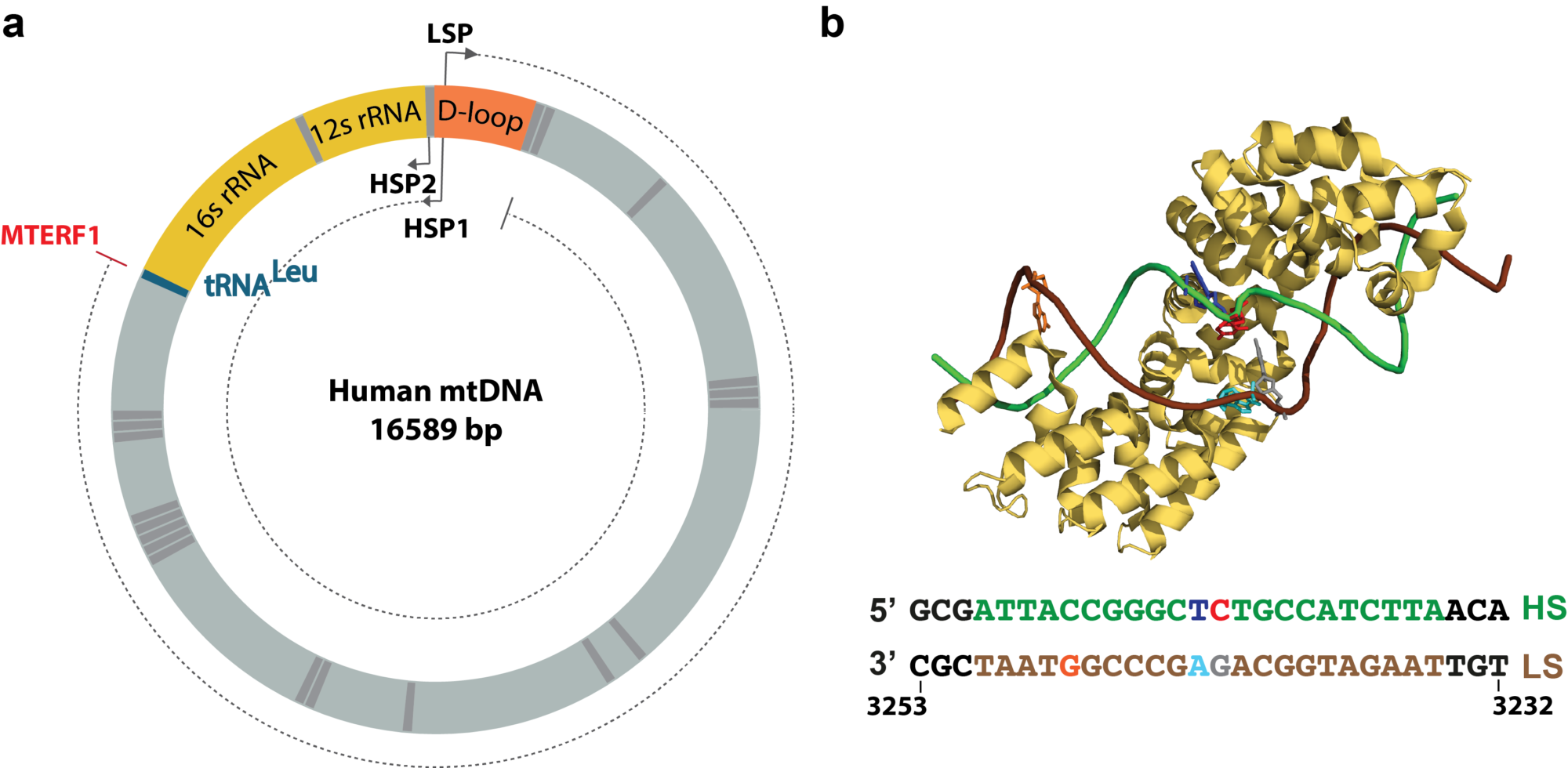
At the end of the gene, the RNAP encounters a termination signal, which comes in the form of, e.g. DNA sequence that encodes a termination signal, such as an RNA hairpin, a protein complex that blocks and dissociates the RNAP. We are particularly interested in understanding protein roadblock mediated transcription termination, such as the one used to terminate human mitochondria transcription, where the mitochondrial termination factor 1 (MTERF1) promotes transcription termination in a directional manner.